Nepsone
Intro
Nepsone is a preclinical-stage pharmaceutical company developing drugs to treat dermatological conditions that arise due to an imbalance in the autonomic nervous system.
Currently, these conditions are believed to originate in the immune system. Nepsone foundational neuroimmunology theory on the origin of psoriasis teaches us that an imbalance in neuropeptide activity in the body leads to these conditions.
Our drugs are designed to utilize the body’s natural mechanisms to bring back balance and alleviate the disease.
Technology Development Fund (Rannís)
Nepsone ehf has been awarded a grant from Tækniþróunarsjóður (Technology Development Fund) of Rannís (The Icelandic Centre for Research).


Pipeline
Nepsone's drug discovery platform can potentially address over 20 indications in total.
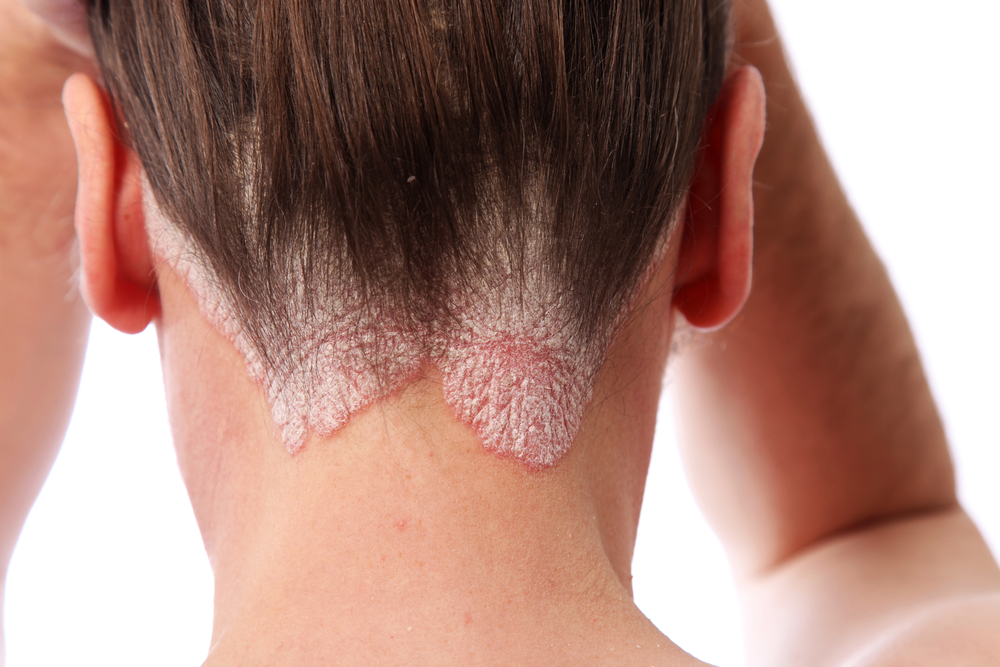
Psoriasis (Lead Indication)
Phase: Preclinical
Modalities: Topical and Systemic
There are several treatments approved for the treatment of psoriasis (e.g. corticosteroids, biologics, light therapy) but no first-in-line treatment has been established yet.
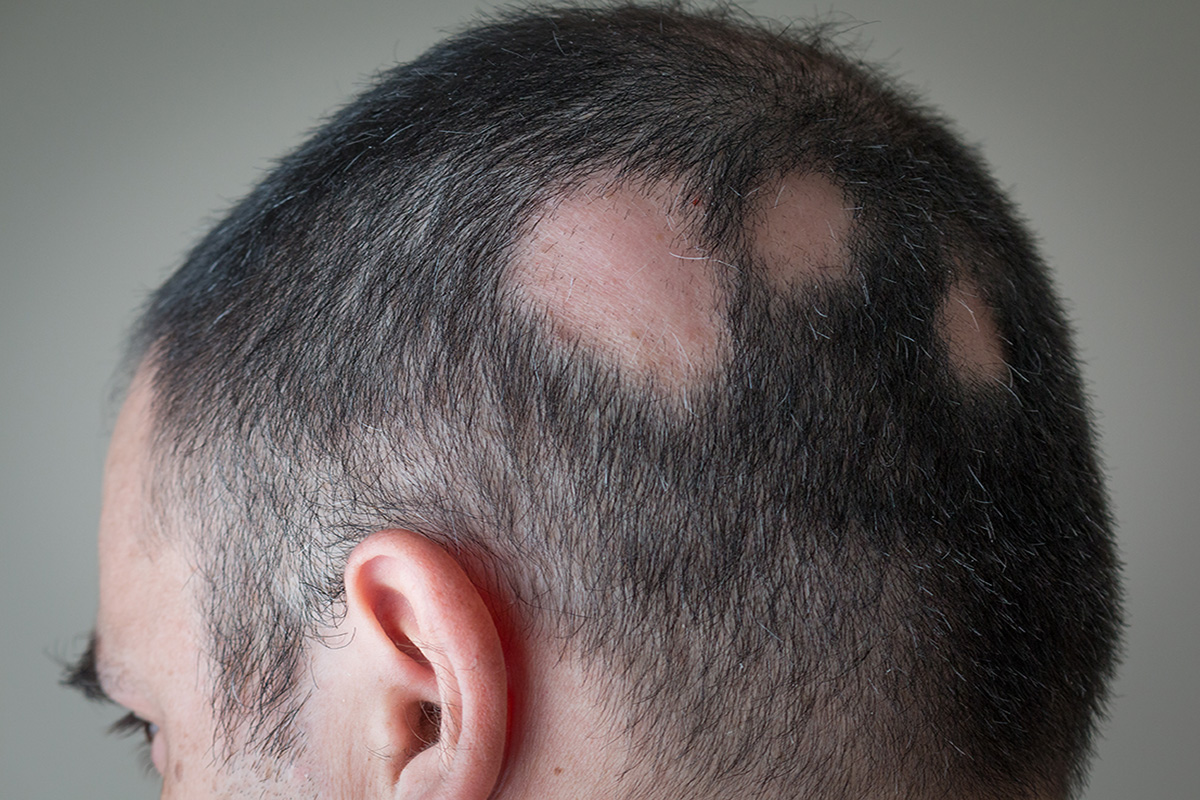
Alopecia Areata
Phase: Research and Discovery
Modality: Topical
The currently available treatment for alopecia areata is the first-in-line Janus kinase inhibitor (JAK) approved by FDA in 2021.
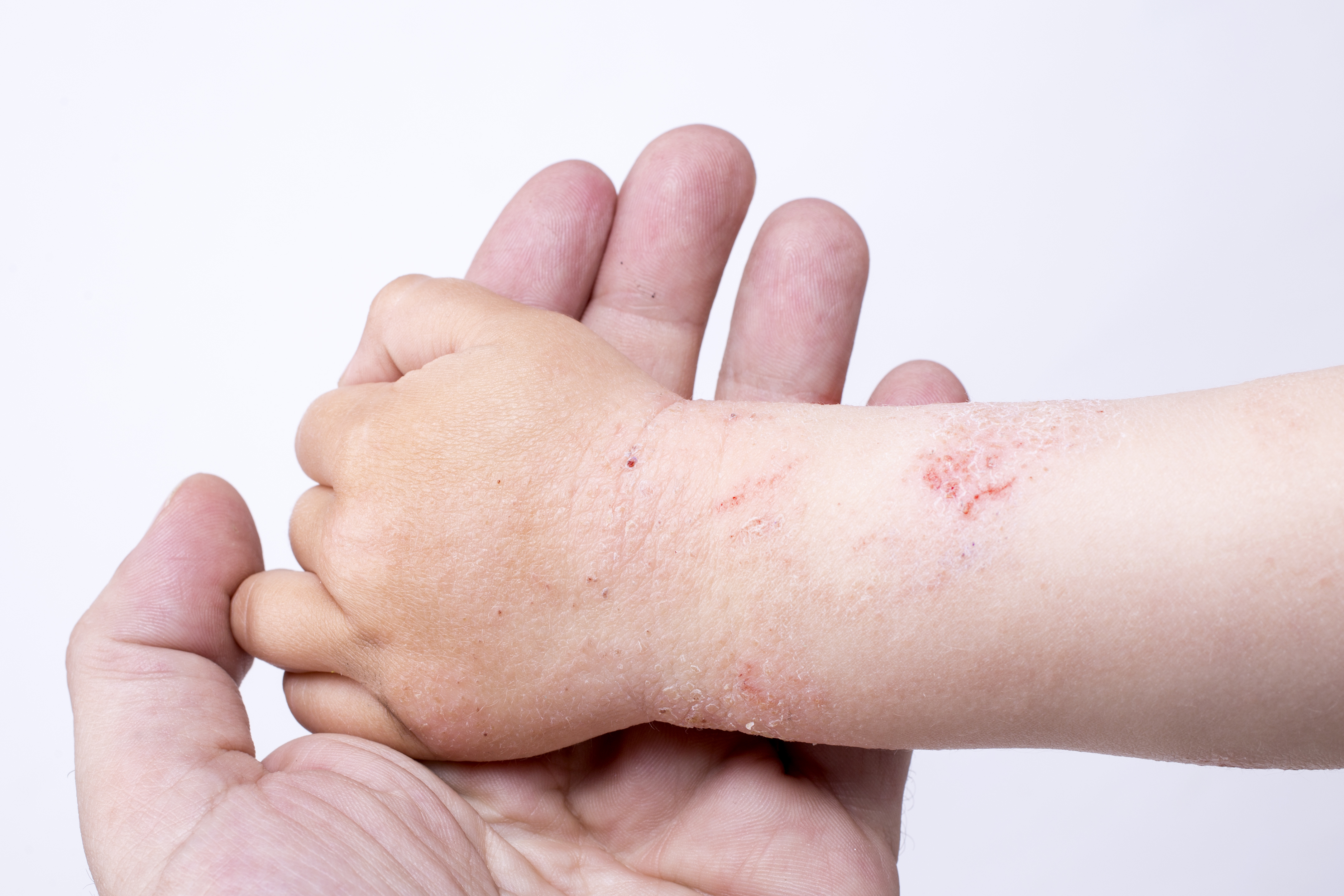
Eczema (Atopic Dermatitis)
Phase: Research and Discovery
Modality: Topical
The current standard-of-care for dermatitis is corticosteroids, biologics or light therapy.
The People of Nepsone

Stefan Jokull Sveinsson
CEO

Bjarki Gudlaugsson
COO

Svandis Kristjansdottir
Head of Clinical Development

Hakon Hrafn Sigurdsson
Head of R&D

Gunhild L Skovgaard
Head of Medical Affairs

Tomas Thorvaldsson
Chairman of the board

Birkir Sveinsson
Founder / Member of the board

Ragnar Bjarnason
Member of the board